Monitoring grafted MSC using a minimally invasive diagnostic concept
Mesenchymal stem cells (MSCs) are one of the most promising sources of cell therapy and regenerative medicine. However, there is lack of in-vivo evidence supporting the interactions of the transplanted MSCs with immune cells. Therefore, in the current project, we are employing liquid biopsy to study safety and immune efficacy of autologous bone marrow MSC therapy.
Blood samples from the patients enrolled in the study will be used after informed consent to evaluate the response/course of cell therapy by characterizing the grafted MSCs in terms of safety, immunomodulation, and role in new bone formation. For this purpose, Mass cytometry is used as a high throughput method of immune phenotyping at single cell level.
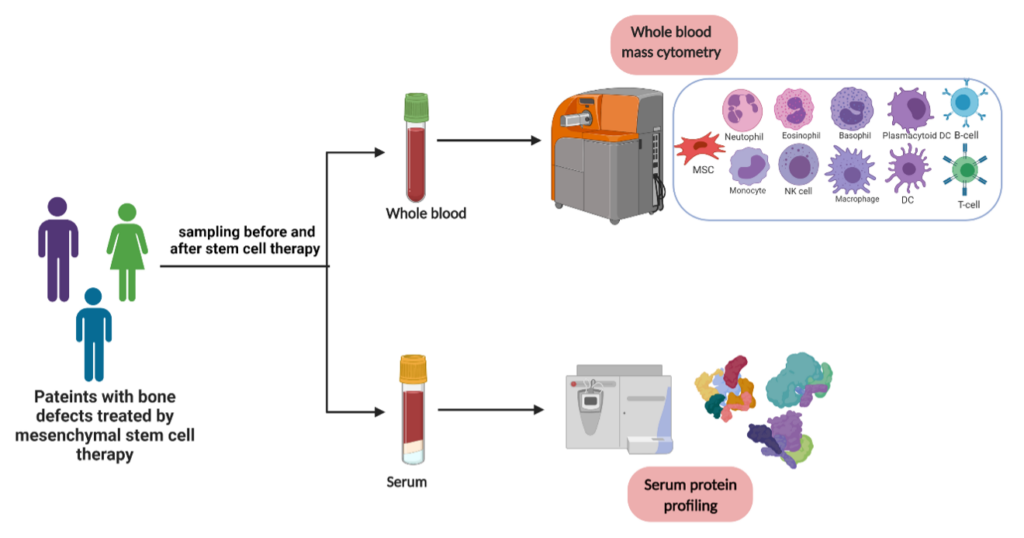
A liquid biopsy is the sampling and analysis of non-solid biological tissue, primarily blood. Use of blood based biomarkers is a non- or minimally invasive procedure. Technological and molecular advances like droplet-based digital PCR, mass cytometry and next generation sequencing technologies, have allowed researchers to purify and analyze liquid biopsy components.
In context of autologous mesenchymal stem cell (MSC) based therapies, liquid biopsy can be employed to assess safety and efficiency of MSC therapy i.e., if transplanted cells are homing to unwanted organs inducing inappropriate differentiation. Blood cells obtained from liquid biopsy can be analyzed with mass cytometry or CyTOF (Cytometry by-time-of flight) where rare-earth metals replace the optical signal detection of conventional flow cytometry.
Additionally, circulating proteins from patient serum samples will be investigated by multiplex assay. This approach can be used to study MSC-immune cell interactions. CyTOF has been used previously to characterize postsurgical immunological state in patients undergoing hip arthroplasty. A surgical immune signature has been found that could lead to patient-specific recovery protocol. Hence, to enable liquid biopsy applications to clinics, understanding how this mechanistic information can be applied to improve patient treatment decisions in regenerative therapies remains one of the key challenges.